Rebinyn® successfully protected patients from bleeds during all major surgeries studied.
aIn a pharmacokinetic assessment of a single dose of Rebinyn® 40 IU/kg upon enrollment in two phase 3 studies, factor levels were evaluated for 1 week after the first dose of Rebinyn® 40 IU/kg. The average levels after 7 days were 16.8% in 6 adults, 14.6% in 3 adolescents, 10.9% in 13 children aged 7 to 12 years, and 8.4% in 12 children up to age 6 years.
EXPERIENCE HIGH FACTOR LEVELS
With a single dose of Rebinyn® 40 IU/kg, adults with less than or equal to 2% Factor 9 levels experienced:



bBased upon a 2.34% increase in factor levels per IU/kg infused in adults receiving a single dose of Rebinyn® 40 IU/kg.
A single dose of Rebinyn® 40 IU/kg was shown to elevate factor levels above their normal levels in pediatric patients.b
In children up to
6 years of age

In children ages
7-12 years

In adolescents ages
13-17 years

bBased on pharmacokinetic assessment of a single dose of Rebinyn® 40 IU/kg after 7 days in 3 adolescents (mean FIX activity 14.6%), 13 children aged 7 to 12 (mean FIX activity 10.9%), and 12 children aged 0-6 (mean FIX activity 8.4%) upon enrollment in the phase 3 trials using 1-stage assay and product-specific standard. All values are geometric mean.
cBased upon a 1.51% increase in factor levels per IU/kg infused in children aged ≤6 receiving a single dose of Rebinyn® 40 IU/kg.
dBased upon a 1.59% increase in factor levels per IU/kg infused in children aged 7-12 receiving a single dose of Rebinyn® 40 IU/kg.
eBased upon a 1.96% increase in factor levels per IU/kg infused in adolescents aged 13-17 receiving a single dose of Rebinyn® 40 IU/kg.
Timothy has severe hemophilia B and uses Rebinyn®.


DID YOU KNOW?
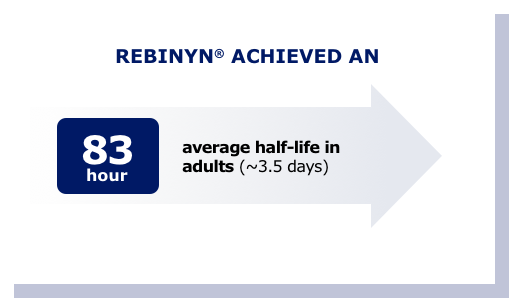
Half-life is the time it takes for the level of factor in the blood to fall by half (50%).

LOOKING FOR RELIABILITY IN YOUR FACTOR 9 TREATMENT?

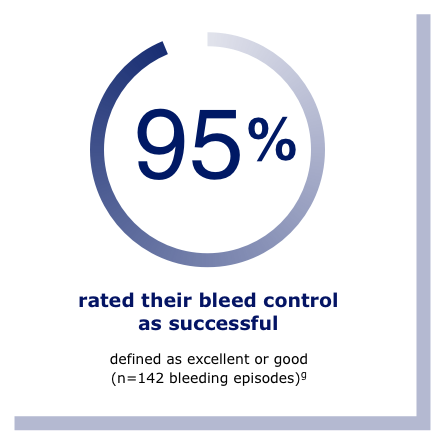
A study of adults and adolescents with hemophilia B showed that Rebinyn® on demand treated 98% of bleeds with 1-2 infusions.f In another study, bleed control was rated successful (defined as excellent or good) in 95% of 142 bleeding episodes.g
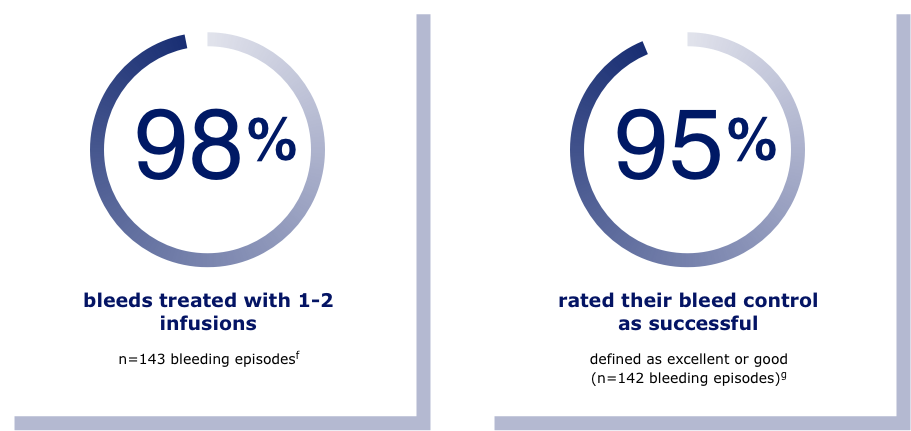
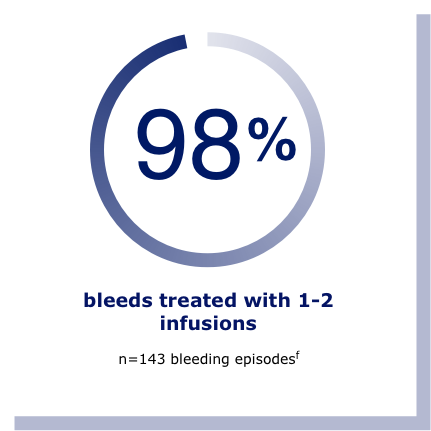
fResults shown are from the on-demand arm of the adolescent/adult clinical study in previously treated patients. In this study, 15 people were treated for on-demand bleeds. In 14 people, there were a total of 143 bleeding episodes.
gResults shown are based on a bleed assessment by either the patient (for home treatment) or the study investigator (for treatment under medical supervision). Bleeds were assessed using a 4-point scale of excellent, good, moderate, or poor.

PROTECTION IN SURGERY

PREVENT BLEEDS WITH PROPHYLAXIS
Learn what once-weekly Rebinyn® can do for you.

HOW IT EXTENDS HALF-LIFE
Rebinyn® uses technology that helps it stay in your body longer, to protect you longer.

![Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)](/content/dam/biopharm/rebinyn/global/rebinyn_logo.png)
