The World Federation of Hemophilia (WFH)c says an extended half-life prophylaxis can allow individuals to keep Factor 9 levels in the non-hemophilia range (greater than 40%) for a significant amount of time.
aFrom a phase 3, open-label trial assessing the safety and efficacy of Rebinyn® after long-term exposure (up to 3 years) in 71 previously treated patients (aged 13 to 70) from paradigm 2 or 3.
bBased on the mean steady-state post-dose peak levels and pre-dose trough levels 168 hours after administered Rebinyn® 40 IU/kg once weekly in previously treated patients (20 adult, 9 adolescent, and 25 pediatric patients).
THE NON-HEMOPHILIA RANGE
THE NON-HEMOPHILIA RANGE

cWFH offers guidelines that inform shared decision-making between patients, caregivers, and healthcare providers based on data from existing peer-reviewed findings.

Timothy has severe hemophilia B and uses Rebinyn®.
dData represent mean steady-state pharmacokinetic profiles from previously treated adolescent/adult patients with moderate to severe hemophilia B (n=9) taking Rebinyn® 40 IU/kg once weekly. Factor 9 levels were within the non-hemophilia range (greater than 40%) for 5.4 days (approximately 80% of the week).
REBINYN® PROPHYLAXIS HELPS PREVENT BLEEDS BEFORE THEY START
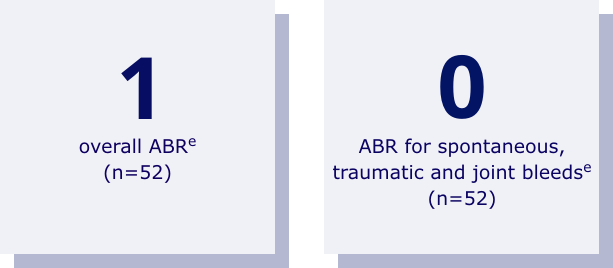
LOW ABR IN ADULTS AND ADOLESCENTS


PROTECT TARGET JOINTS

ABR=annualized bleeding rate.
eFrom a phase 3, open-label trial assessing the safety and efficacy of Rebinyn® after long-term exposure (up to 3 years) in 71 previously treated patients (aged 13 to 70) from paradigm 2 or 3.
f20 target joints were reported in 13 patients in the 40 IU/kg once weekly arm at baseline, and 18 out of 20 (90%) of these target joints were considered resolved at the end of the main phase. There were 2 target joints at the start of the extension trial in patients in the 40 IU/kg once-weekly arm. Upon conclusion of the extension phase, both of these target joints were resolved, as measured by International Society on Thrombosis and Haemostasis (ISTH) target joint criteria. The definition of target joint from the ISTH is greater than or equal to 3 spontaneous bleeds into the joint within a consecutive 6-month period. When there have been less than or equal to 2 bleeds into the joint within a consecutive 12-month period, the joint is no longer considered a target joint.
WITH REBINYN® PROPHYLAXIS, CHILDREN 12 AND UNDER ACHIEVED A YEARLY BLEED RATE OF:g
6 years and under (n=12)



7 to 12 years (n=13)



g25 previously treated children 0 to 12 years old received routine prophylactic administration of Rebinyn® 40 IU/kg once weekly for 52 week.
DID YOU KNOW?
Rebinyn® prophylaxis only needs to be taken once per week. Simple dosing may be easier to remember and fit into your routine.h
hDosing regimen may be adjusted based on individual patient’s bleeding pattern, and physical activity.

SIMPLIFIED DOSING -
FLEXIBLE STORAGE
Rebinyn® offers simple, once-weekly dosing, and flexible storage.
CHOOSING THERAPIES
See Rebinyn® PK compared to other therapies
![Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)](/content/dam/biopharm/rebinyn/global/rebinyn_logo.png)
