Studies show that Rebinyn® provides effective bleed control for children, adolescents, and adults.
GET EFFECTIVE PROTECTION IN SURGERY
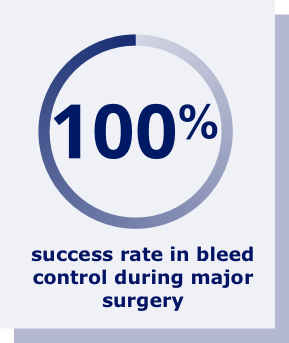
Rebinyn® provided
100% success rate in bleed control during surgery.a
aResults shown are from the surgery clinical study, which included 13 previously treated adolescent and adult patients. Patients received 1 infusion of Rebinyn® 80 IU/kg on the day of their surgeries. At the doctor’s discretion, patients received infusions of Rebinyn® 40 IU/kg for up to 3 weeks after surgery. Across 13 surgical procedures (9 major), the success rate in bleed control during surgery was evaluated on a four-point scale of excellent, good, moderate, or poor. Treatment success was defined as excellent or good bleed control.
BLEED PROTECTION YOU CAN COUNT ON

In all major and minor surgeries and procedures studied, doctors rated Rebinyn® as excellent or good in protecting their patients from bleeds.a
REBINYN® PROVIDED

THE POWER TO CONTROL BLEEDS
THE POWER TO CONTROL BLEEDS

RESOURCES FOR YOU
These materials can help you stay educated on bleeding disorders.

DESIGNED WITH SAFETY IN MIND
In clinical studies, Rebinyn® was shown to be safe and effective.
![Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)](/content/dam/biopharm/rebinyn/global/rebinyn_logo.png)