Your doctor may adjust your dosing regimen based on your bleeding pattern and physical activity.
APPROVED
FOR ONCE-WEEKLY
PROPHYLAXIS
To prevent or reduce the number of bleeding episodes in people with hemophilia B
Be ready for the unexpected
Once-weekly Rebinyn® helps you keep Factor 9 levels higher for longer.a

APPROVED
FOR ONCE-WEEKLY
PROPHYLAXIS
To prevent or reduce the number of bleeding episodes in people with hemophilia B
Be ready for the
unexpected
Once-weekly Rebinyn® helps
you keep Factor 9 levels higher for longer.a

aRebinyn® achieved and maintained higher factor levels than recombinant Factor 9 based upon a phase 1 study comparing 25, 50, and 100 IU/kg single doses of Rebinyn® to a 50 IU/kg single dose of standard half-life recombinant Factor 9 in 7 adults and a 50 IU/kg dose of plasma-derived Factor 9 in 8 adults. For Rebinyn®, estimated average Factor 9 activity is adjusted to a dose of 50 IU/kg. Incremental recovery at 30 minutes (IR30) and half-life were higher and longer with Rebinyn® than recombinant Factor 9 (IR30 0.0131 vs 0.0068 (IU/mL)/(IU/kg) and half-life 93 vs 19 hours). The clinical relevance of these pharmacokinetic differences is unknown. Incremental recovery is the increase in plasma concentration per IU/kg of factor administered. Half-life is the time it takes for the level of factor in the blood to fall by half (50%).
SIMPLE ONCE-WEEKLY DOSING FOR ROUTINE PROPHYLAXIS:
MAINTAINING FACTOR LEVELS BETWEEN DOSES

The World Federation of Hemophilia (WFH)b says an extended half-life prophylaxis can allow individuals to keep Factor 9 levels in the non-hemophilia range (greater than 40%) for a significant amount of time.
The World Federation of Hemophilia (WFH)b says an extended half-life prophylaxis can allow individuals to keep Factor 9 levels in the non-hemophilia range (greater than 40%) for a significant amount of time.
bWFH offers guidelines that inform shared decision-making between patients, caregivers, and healthcare providers based on data from existing peer-reviewed findings.
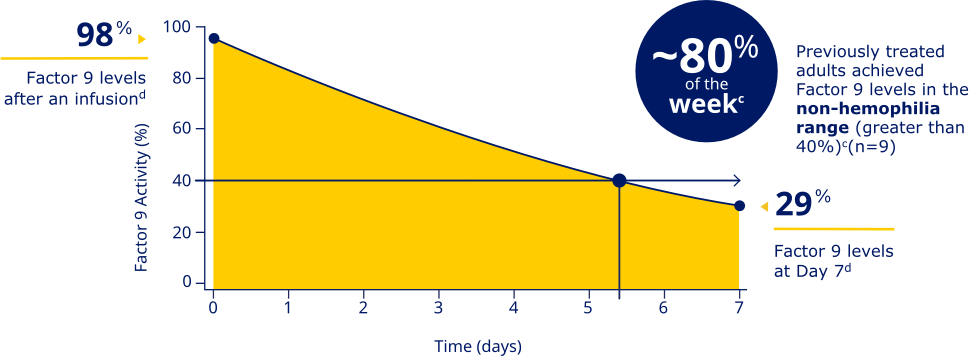
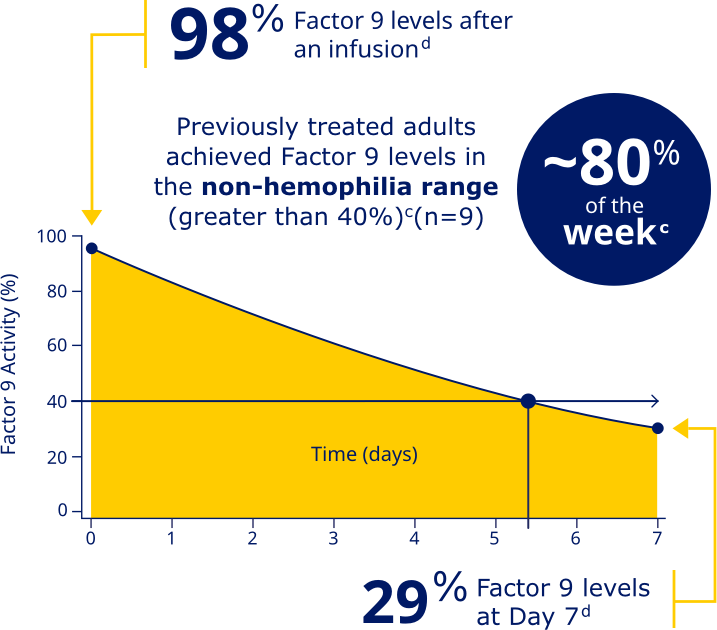
REBINYN® HELPS YOU ACHIEVE HIGH FACTOR LEVELS SO YOU
CAN BE READY FOR THE UNEXPECTEDc

Previously treated adults achieved Factor 9 levels in the non-hemophilia range (greater than 40%)c
IN ADULTS THE
HALF-LIFEe WAS
Factor 9 levels after an infusion were
98% (N=20), and Factor 9 levels
at Day 7 were 29% (N=20).d
IN ADOLESCENTS THE
HALF-LIFEe WAS
Factor 9 levels after an infusion were
83% (N=9), and Factor 9 levels
at Day 7 were 24%d
IN ADULTS THE
HALF-LIFEe WAS
Factor 9 levels after an infusion were
98% (N=20), and Factor 9 levels
at Day 7 were 29% (N=20).d

IN ADOLESCENTS THE
HALF-LIFEe WAS
Factor 9 levels after an infusion were
83% (N=9), and Factor 9 levels
at Day 7 were 24%d
cData represent mean steady-state pharmacokinetic profiles from previously treated adolescent/adult patients with moderate to severe hemophilia B (n=9) taking repeated doses of Rebinyn® 40 IU/kg once weekly. Factor 9 levels were within the non-hemophilia range (greater than 40%) for 5.4 days (approximately 80% of the week).
dBased on the mean steady-state post-dose peak levels and pre-dose trough levels 168 hours after administered Rebinyn® 40 IU/kg once weekly in previously treated patients (20 adult and 9 adolescent patients).
eHalf-life: Time required for Factor 9 to decline to half its value.
fBased on analysis using a 1-stage assay in patients (n=6) aged 18 and older, the half-life at steady state was 115 hours following once-weekly (40 IU/kg) dosing; in patients (n=3) aged 13 to 17, the half-life at steady state was 103 hours. Following single-dose administration (40 IU/kg) in the same patient population, the half-life was 83 hours (adults) and 89 hours (adolescents).
ESTABLISHED SAFETY PROFILE
ESTABLISHED SAFETY PROFILE
Clinical trials over
13 YEARS
showed 0 inhibitors and 0 blood clots in previously treated patients.
The formation of inhibitors (neutralizing antibodies) to Factor 9 has occurred following Rebinyn®. Common adverse reactions (incidence ≥1%) in PUPs reported in clinical trials for Rebinyn® included Factor 9 inhibitors. The use of Factor 9-containing products has been associated with blood clots.

WE'RE HERE TO HELP
Wondering about Rebinyn® or living with hemophilia B? Our Rare Blood Community Liaisons (RBCLs) are here to answer your questions and connect you with the resources you need.

WE'RE HERE TO HELP
Wondering about Rebinyn® or living with hemophilia B? Our Rare Blood Community Liaisons (RBCLs) are here to answer your questions and connect you with the resources you need.
![Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)](/content/dam/biopharm/rebinyn/global/rebinyn_logo.png)