Data from Rebinyn® PI, 2022; Alprolix® PI, 2020; Idelvion® PI, 2021; BeneFIX® PI, 2021; Ixinity® PI, 2021; and Rixubis® PI, 2020.
IS REBINYN® RIGHT FOR YOU?
It’s important to consider all your options when choosing a Factor 9 treatment. See different Factor 9 therapies; Rebinyn® may be the one for you.
It’s important to consider all your options when choosing a Factor 9 treatment. See how Rebinyn® compares to others—it may be the one for you.

All images of hemophilia B patients shown are for illustrative purposes only.
IS REBINYN® RIGHT
FOR YOU?
It’s important to consider all your options when choosing a Factor 9 treatment. See different Factor 9 therapies; Rebinyn® may be the one for you.

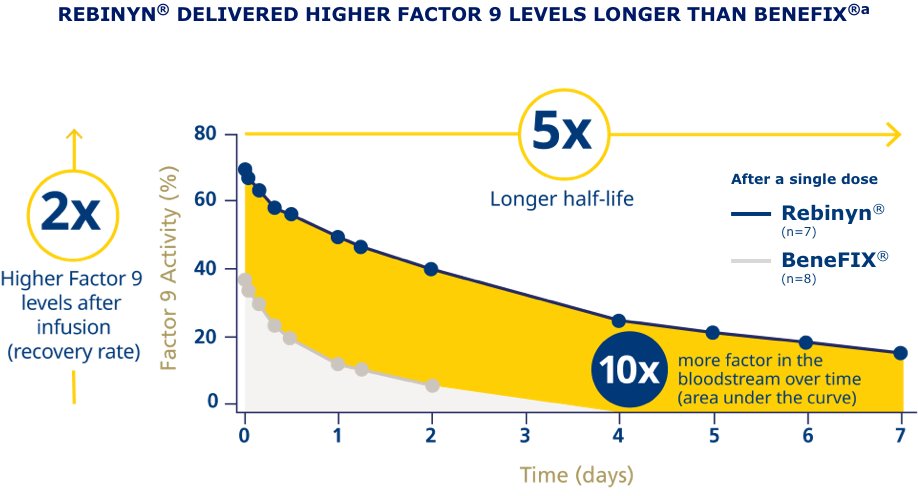
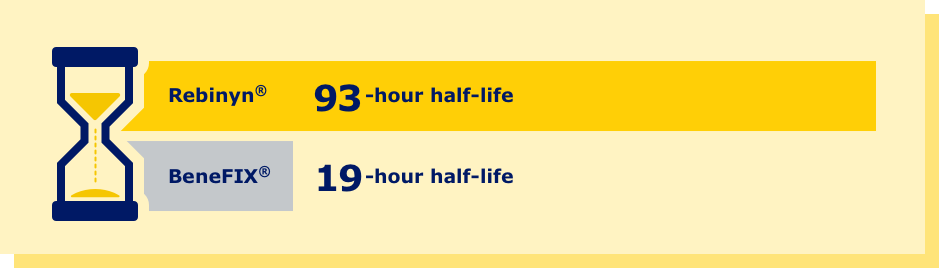
pdFIX=plasma-derived Factor 9; rFIX=recombinant Factor 9; SHL=standard half-life.
aPhase 1 trial comparing pharmacokinetics of Rebinyn® with SHL Factor 9 products. Based upon a phase 1 study of patients administered 1 of 3 doses of Rebinyn® (25, 50, or 100 IU/kg) compared with 1 dose of their prior SHL rFIX (n=7) or pdFIX (n=8) at the same dose using a 1-stage assay. Estimated mean pharmacokinetic parameters are adjusted to a dose of 50 IU/kg. Differences were similar in comparison of Rebinyn® to pdFIX (half-life 93 vs 18 hours, 5.2x, P<0.001; AUC 70 vs 9 U x h/mL, 8x, P<0.001) and in comparison of Rebinyn® to rFIX (half-life 93 vs 19 hours, P<0.001; AUC 72 vs 7 U x h/mL, P<0.001). The clinical relevance of these PK differences is unknown.
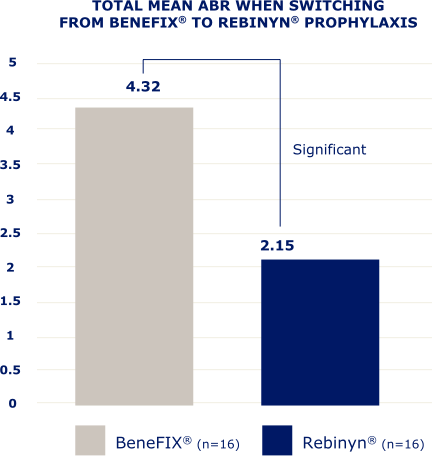
IN A RETROSPECTIVE, OBSERVATIONAL, REAL-WORLD STUDY OF 42 PATIENTS,b FEWER BLEEDS WERE SEEN IN PATIENTS WHO SWITCHED TO REBINYN®
IN A RETROSPECTIVE, OBSERVATIONAL, REAL-WORLD STUDY OF 42 PATIENTS,b FEWER BLEEDS WERE SEEN IN PATIENTS WHO SWITCHED TO REBINYN®

ABR=annualized bleeding rate.
bBased on a retrospective, observational, real-world study of 42 patients with hemophilia B (5 patients were less than 18 years old) who switched from either BeneFIX® or Alprolix® prophylaxis to Rebinyn® prophylaxis using published Canadian Bleeding Disorders Registry (CBDR) data. Patients had to be on a previous therapy for at least 6 months and have at least 6 months of follow-up with Rebinyn®. CBDR formulary required a switch from Alprolix® to Rebinyn®, and patients had the option to switch from BeneFIX® to Rebinyn®. The study took place in part during the COVID-19 pandemic which limited attendance at hemophilia clinics for physical examinations. Total mean ABR is based on intrapatient bleeding, which is the bleeding rate in each patient before and after switching from BeneFIX® or Alprolix® prophylaxis to Rebinyn® prophylaxis.
FOR ON-DEMAND TREATMENT, PATIENTS PREVIOUSLY TREATED WITH MULTIPLE STANDARD HALF-LIFE (SHL) DOSES NEEDED UP TO 80% LESS FACTOR 9 WITH REBINYN®c
FOR ON-DEMAND TREATMENT, PATIENTS PREVIOUSLY TREATED WITH MULTIPLE STANDARD HALF-LIFE (SHL) DOSES NEEDED UP TO 80% LESS FACTOR 9 WITH REBINYN®c

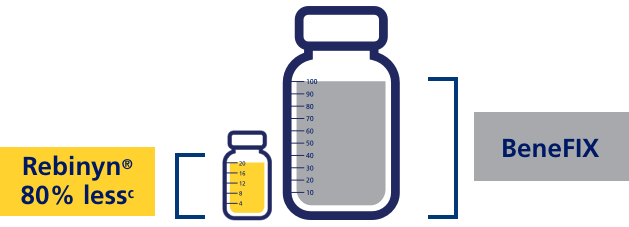
cIn a phase 3 study, the efficacy of Rebinyn® in adults/adolescents was evaluated. The on-demand arm included 15 patients; 1 patient had no bleeds, and the other 14 patients received Rebinyn® 40 IU/kg as single dose for the treatment of bleeds. Patients received on-demand treatment for 28 weeks. 7 patients controlled all bleeds (62 bleeds) with a single 40 IU/kg dose of Rebinyn®. The remaining 7 patients (81 bleeds) required ≥2 doses to control at least one of their bleeds. Of those 7, 4 patients who were previously treated with multiple high doses (2-3 doses of 60 IU/kg or 80 IU/kg) were able to control 71% of their bleeds with a single dose and used 58%-80% less FIX while using Rebinyn® 40 IU/kg. 243 IU/kg with pdFIX vs 47.5 IU/kg with Rebinyn®. This was reported in one patient.
COMPARED WITH SHL BENEFIX® AND PLASMA-DERIVED FIX (pdFIX), REBINYN® MAY REQUIRE FEWER INFUSIONS PER EPISODE FOR MILD TO MODERATE BLEEDS, SEVERE BLEEDS, AND DURING AND AFTER SURGERYd,e
COMPARED WITH SHL BENEFIX® AND PLASMA-DERIVED FIX (pdFIX), REBINYN® MAY REQUIRE FEWER INFUSIONS PER EPISODE FOR MILD TO MODERATE BLEEDS, SEVERE BLEEDS, AND DURING AND AFTER SURGERYd,e

Estimated number of doses and amount of Factor 9 per episode, based on pharmacokinetic (PK) modeling (a mathematical simulation):
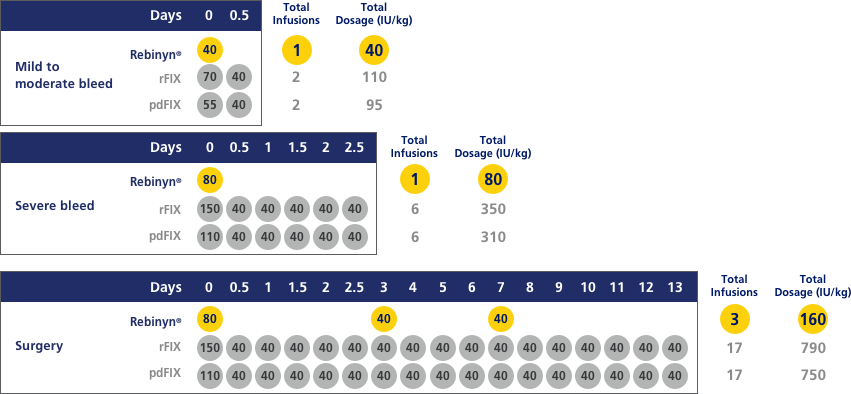
dA single dose of Rebinyn® 40 IU/kg should be enough for minor and moderate bleeds. Your doctor may recommend additional doses of 40 IU/kg.
eBased on a pharmacokinetic (PK) modeling to World Federation of Hemophilia (WFH) guidelines. Simulated results based on phase 1 PK study of Rebinyn® (n=15), recombinant FIX (rFIX) (n=7), and plasma-derived FIX (pdFIX) (n=8).
REBINYN® DELIVERED HIGHER FACTOR 9 LEVELS LONGER THAN ALPROLIX®f
REBINYN® DELIVERED HIGHER FACTOR 9 LEVELS LONGER THAN ALPROLIX®f
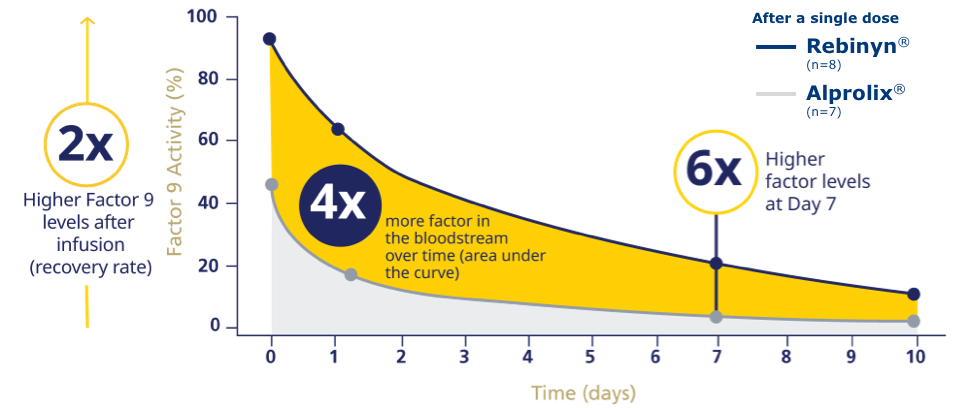
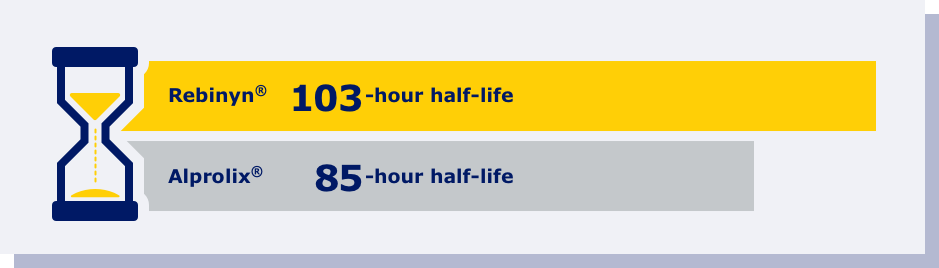
fPhase 1 trial comparing pharmacokinetics of Rebinyn® with Alprolix®. Based upon a phase 1 study of 15 patients administered a single dose of Rebinyn® 50 IU/kg compared with a single dose of Alprolix® 50 IU/kg using both 1-stage (shown above) and chromogenic assays. The standard Alprolix® dose of 50 IU/kg was administered for both products to allow for comparison of dose-dependent parameters; dose normalized to 50 IU/kg to reflect minor differences in dose administered. Geometric mean half-life was also prolonged (Rebinyn®: 103.2 hours, Alprolix®: 84.9 hours). All comparisons were significant (P<0.0001) for all assays. The clinical relevance of these pharmacokinetic differences is unknown.
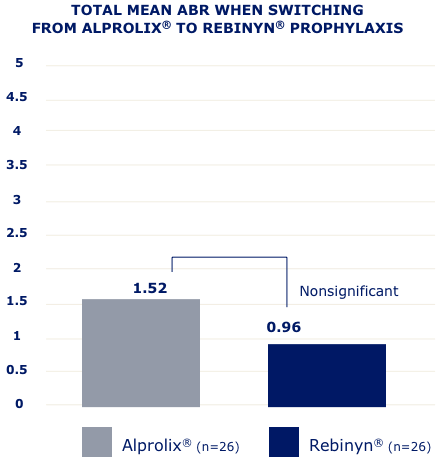
IN A RETROSPECTIVE, OBSERVATIONAL, REAL-WORLD STUDY OF 42 PATIENTS,g FEWER BLEEDS WERE SEEN IN PATIENTS WHO SWITCHED TO REBINYN®
IN A RETROSPECTIVE, OBSERVATIONAL, REAL-WORLD STUDY OF 42 PATIENTS,g FEWER BLEEDS WERE SEEN IN PATIENTS WHO SWITCHED TO REBINYN®



ABR=annualized bleeding rate.
gBased on a retrospective, observational, real-world study of 42 patients with hemophilia B (5 patients were less than 18 years old) who switched from either BeneFIX® or Alprolix® prophylaxis to Rebinyn® prophylaxis using published Canadian Bleeding Disorders Registry (CBDR) data. Patients had to be on a previous therapy for at least 6 months and have at least 6 months of follow-up with Rebinyn®. CBDR formulary required a switch from Alprolix® to Rebinyn®, and patients had the option to switch from BeneFIX® to Rebinyn®. The study took place in part during the COVID-19 pandemic which limited attendance at hemophilia clinics for physical examinations. Total mean ABR is based on intrapatient bleeding, which is the bleeding rate in each patient before and after switching from BeneFIX® or Alprolix® prophylaxis to Rebinyn® prophylaxis.
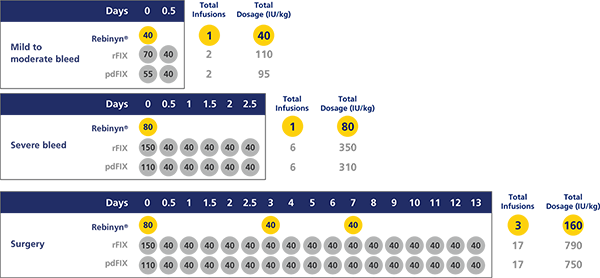
FOR ON-DEMAND TREATMENT, REBINYN® MAY REDUCE THE NEED FOR ADDITIONAL INFUSIONS VS EXTENDED HALF-LIFE ALPROLIX®h,i
FOR ON-DEMAND TREATMENT, REBINYN® MAY REDUCE THE NEED FOR ADDITIONAL INFUSIONS VS EXTENDED HALF-LIFE ALPROLIX®h,i

Estimated number of doses and amount of Factor 9 per episode, based on pharmacokinetic (PK) modeling (a mathematical simulation)
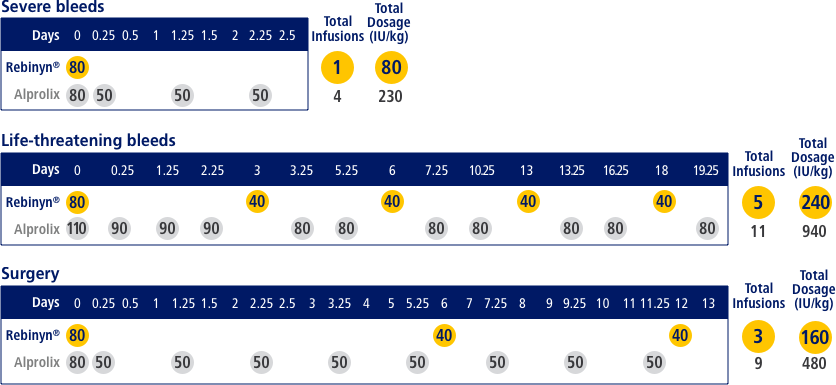
hA single dose of Rebinyn® 40 IU/kg should be enough for minor and moderate bleeds. Your doctor may recommend additional doses of 40 IU/kg.
iBased on pharmacokinetic modeling to WFH guidelines. Simulated results based on phase 1 pharmacokinetic studies of Rebinyn® (n=15) and Alprolix® (n=15).
HERE’S HOW REBINYN® COMPARES WITH OTHER FIX PRODUCTS BASED ON SELECTED KEY ATTRIBUTES
HERE’S HOW REBINYN® COMPARES WITH OTHER FIX PRODUCTS BASED ON SELECTED KEY ATTRIBUTES

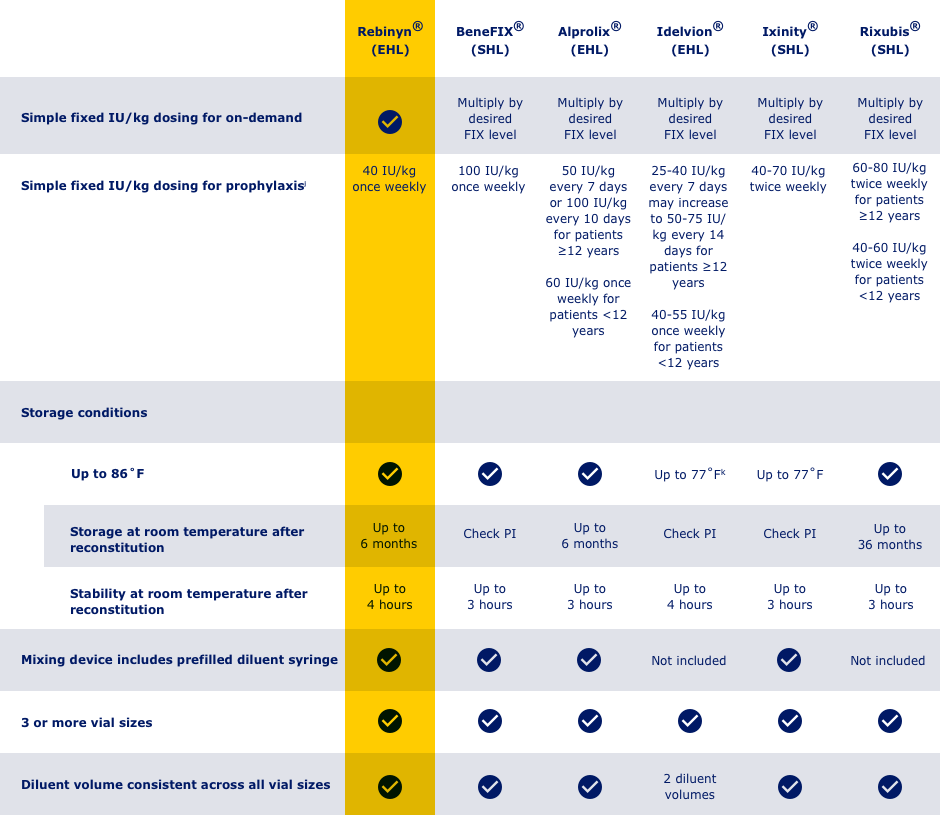
jAdjust dosing regimen based on individual response
k250 IU strength only: store at 36-46˚F
Not intended to be a comparison of efficacy and safety.
PREVENT BLEEDS WITH PROPHYLAXIS
Learn what once-weekly prophylaxis can do for you.

WE'RE HERE TO HELP
Connect with a Rare Blood Community Liaison (RBCL) for hemophilia-related resources.

![Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)](/content/dam/biopharm/rebinyn/global/rebinyn_logo.png)