Factor 9 levels after an infusion were
98% (N=20), and Factor 9 levels
at Day 7 were 29% (N=20).b
PREVIOUSLY TREATED ADULTS ACHIEVED FACTOR 9 LEVELS IN THE NON-HEMOPHILIA RANGE (GREATER THAN 40%)a

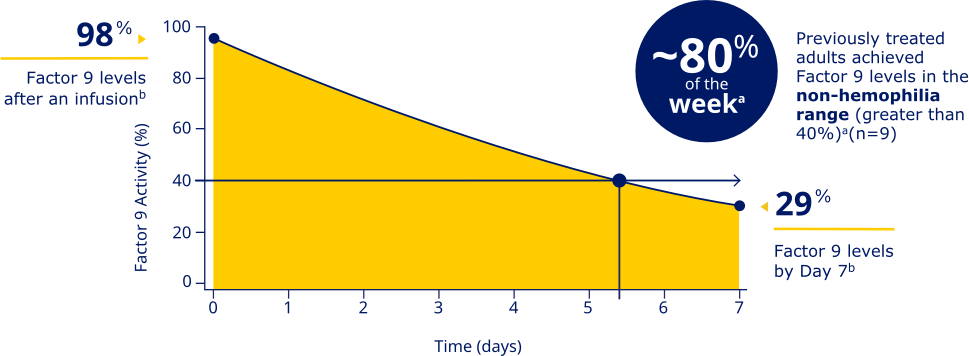
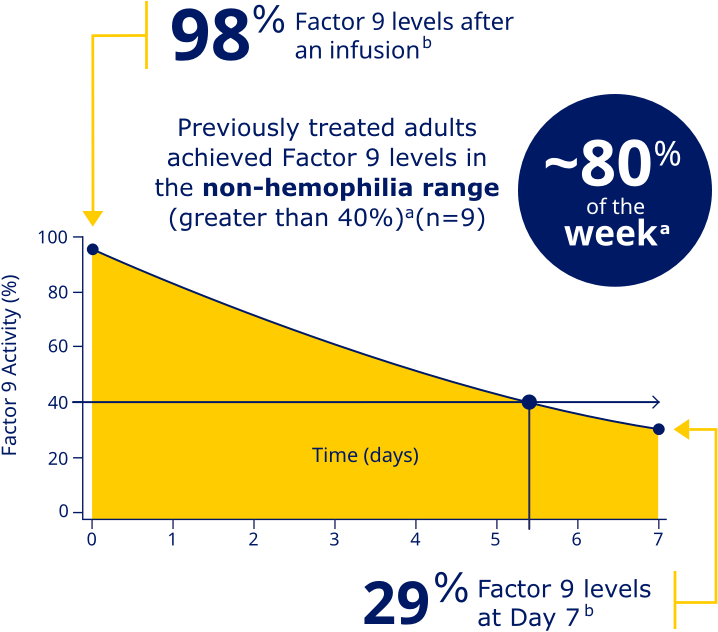
aData represent mean steady-state pharmacokinetic profiles from previously treated adolescent/adult patients with moderate to severe hemophilia B (n=9) taking repeated doses of Rebinyn® 40 IU/kg once weekly. Factor 9 levels were within the non-hemophilia range (greater than 40%) for 5.4 days (approximately 80% of the week).
bBased on the mean steady-state post-dose peak levels and pre-dose trough levels 168 hours after administered Rebinyn® 40 IU/kg once weekly in previously treated patients (20 adult and 9 adolescent patients).
IN ADULTS, THE
HALF-LIFEc WAS
IN ADOLESCENTS,
THE HALF-LIFEc WAS
Factor 9 levels after an infusion were
83% (N=9), and Factor 9 levels
at Day 7 were 24%b
IN ADULTS, THE
HALF-LIFEc WAS
Factor 9 levels after an infusion were
98% (N=20), and Factor 9 levels
at Day 7 were 29% (N=20).b

IN ADOLESCENTS,
THE HALF-LIFEc WAS
Factor 9 levels after an infusion were
83% (N=9), and Factor 9 levels
at Day 7 were 24%b
cHalf-life: Time required for Factor 9 to decline to half its value.
dBased on analysis using a 1-stage assay in patients (n=6) aged 18 and older, the half-life at steady state was 115 hours following once-weekly (40 IU/kg) dosing; in patients (n=3) aged 13 to 17, the half-life at steady state was 103 hours. Following single-dose administration (40 IU/kg) in the same patient population, the half-life was 83 hours (adults) and 89 hours (adolescents).
MAINTAINING FACTOR LEVELS BETWEEN DOSES
MAINTAINING FACTOR LEVELS BETWEEN DOSES

The World Federation of Hemophilia (WFH)e says an extended half-life prophylaxis can allow individuals to keep Factor 9 levels in the non-hemophilia range (greater than 40%) for a significant amount of time.
eWFH offers guidelines that inform shared decision-making between patients, caregivers, and healthcare providers based on data from existing peer-reviewed findings.

Timothy has severe hemophilia B and uses Rebinyn®.
fData represent mean steady-state pharmacokinetic profiles from previously treated adolescent/adult patients with moderate to severe hemophilia B (n=9) taking repeated doses of Rebinyn® 40 IU/kg once weekly. Factor 9 levels were within the non-hemophilia range (greater than 40%) for 5.4 days (approximately 80% of the week).
HIGHER LEVELS WITH REBINYN® CAN HELP KIDS BE READY FOR THE UNEXPECTED

HIGH FACTOR 9 LEVELS IN CHILDREN 12 YEARS OR YOUNGER
gMean steady-state pre-dose trough levels and post-dose peak levels across the clinical trials for all previously treated children receiving Rebinyn® 40 IU/kg once weekly. Based on an analysis using a 1-stage assay of previously treated children aged 0 to 12 years old (N=25). Children were stratified into 2 groups: ≤6 years old (n=12) and 7 to 12 years old (n=13). All patients received a fixed dose of 40 IU/kg of Rebinyn® intravenously once weekly for prophylaxis.
hBased on single-dose pharmacokinetic parameters of Rebinyn® 40 IU/kg in children aged ≤6 (n=12) and 7 to 12 years (n=13). A single-dose pharmacokinetic assessment was conducted after administration of the first dose of Rebinyn®. The last pharmacokinetic sample was collected 1 week later, just prior to administration of the second dose.
DID YOU KNOW?
Half-life is the time it takes for the level of factor in the blood to fall by half (50%).

INTERESTED IN TRYING REBINYN®?

To learn more about our free trial prescription program, please call 1-844-668-6732 to speak with NovoCare®.

Commercially insured patients only. Eligibility and restrictions apply.
PREVENT BLEEDS
WITH REBINYN®
Learn what once-weekly Rebinyn® can do for you.

CONSIDERING A SWITCH?
See how Factor 9 therapies compare on features that matter to you—from storage conditions to infusion time.
![Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)](/content/dam/biopharm/rebinyn/global/rebinyn_logo.png)